Description
Description
Clodronate disodium is a non-amino, chloro-containing bisphosphonate. Chemically, clodronate disodium is (dichloromethylene) diphosphonic acid disodium salt and is manufactured from the tetrahydrate form.
Active Ingredients
Each mL contains 60 mg clodronate disodium (as clodronate disodium tetrahydrate), EP. Contains no preservatives.
OSPHOS Injection Indications
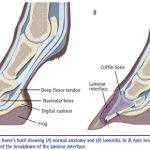
For the control of clinical signs associated with navicular syndrome in horses.
Dosage and Administration
Administer by intramuscular injection. Divide the total volume of OSPHOS Injection to be administered evenly into three (3) separate injection sites.The recommended dose is 1.8 mg clodronate disodium/kg body weight (3 mL per 100 kg body weight) up to a maximum dose of 900 mg (15 mL) per horse.
Clinical improvement is most evident at 2 months post-treatment (see EFFICACY). If there is no response to initial therapy, the horse should be re-evaluated.
For horses that initially respond to OSPHOS Injection but do not maintain their clinical improvement for 6 months, OSPHOS Injection may be re-administered at 3 to 6 month intervals based on recurrence of clinical signs. For horses that respond to OSPHOS Injection and maintain clinical improvement for 6 months, OSPHOS Injection should be re-administered after clinical signs recur.
Contraindications
– Do not use OPSHOS Injection in horses with known hypersensitivity to clodronate disodium.
– Do not use this product in horses with impaired renal function or with a history of renal disease.
CAUTIONS:
– The effect of bisphosphonates on the skeleton of growing horses has not been studied, therefore use in horses less than 4 years of age is not recommended.
– The safe use of OSPHOS Injection has not been evaluated in breeding horses or pregnant or lactating mares. Bisphosphonates have been shown to cause fetal developmental abnormalities in laboratory animals and may be excreted in milk. Therefore, this drug should not be used in pregnant or lactating mares, or breeding horses.
– Ensure that horses are adequately hydrated when administering the product.
– Concurrent administration of other potentially nephrotoxic drugs should be approached with caution and renal function should be monitored (see ADVERSE REACTIONS).
– Caution should be used when administering bisphosphonates to horses with conditions affecting mineral or electrolyte homeostasis (e.g., hyperkalemic periodic paralysis, hypocalcemia, etc.) since bisphosphonates affect plasma concentrations of calcium, magnesium and potassium, immediately post-treatment, with effects lasting up to several hours.
– Avoid overdosing with this product as increased bone fragility has been observed in laboratory animals and humans treated with bisphosphonates at high doses or for long periods of time.
Warnings
– Keep out of reach of children.
– Not for use in horses that are to be slaughtered for use in food.
– Care should be taken when handling the product to avoid self-injection, especially by pregnant women. In case of accidental
self-injection, consult a physician immediately.
– Avoid contact with skin or eyes. Accidental spillage on the skin or eyes should be washed off with water.
Adverse Reactions
Overview:
As a class, bisphosphonates may be associated with gastrointestinal and renal toxicity. Renal and gastrointestinal adverse reactions may be associated with high plasma concentrations of the drug. The administration of bisphosphonates has been associated with abdominal pain (colic), discomfort, and agitation in horses. Clinical signs usually occur shortly after drug administration and may be associated with alterations in intestinal motility. In horses treated with the recommended dose of OSPHOS Injection, these clinical signs (see Tables 1 and 4) usually began within 2 hours of treatment.
Field trial data:
One hundred forty-six horses (111 OSPHOS Injection, 35 saline control) of various breeds, 4 to 22 years of age, and weighing 367-601 kg (807 to 1322 lb) were included in the field study safety analysis.
Following treatment on Day 0, 10 horses had clinical signs of discomfort or nervousness, cramping, pawing, and/or colic within 2 hours post-treatment. One horse experiencing colic and hives required treatment with flunixin and dexamethasone to resolve clinical signs. In 8 of the 10 horses, 10 to 15 minutes of hand walking resulted in resolution of clinical signs. In one horse, clinical signs resolved without hand walking. Three additional horses experienced lip licking, yawning, and/or head shaking. Adverse reactions occurring within 2 hours post-treatment with OSPHOS Injection or the saline control are summarized in Table 1.











Reviews
There are no reviews yet.